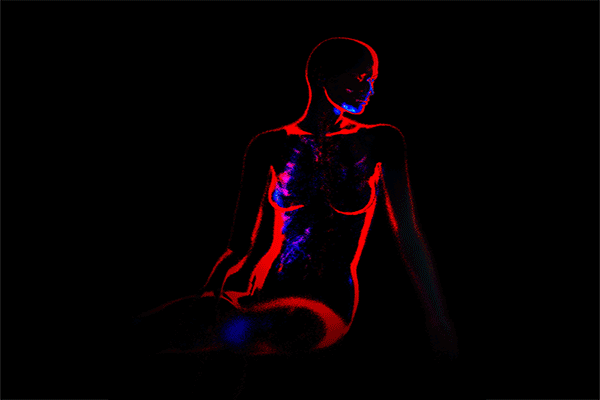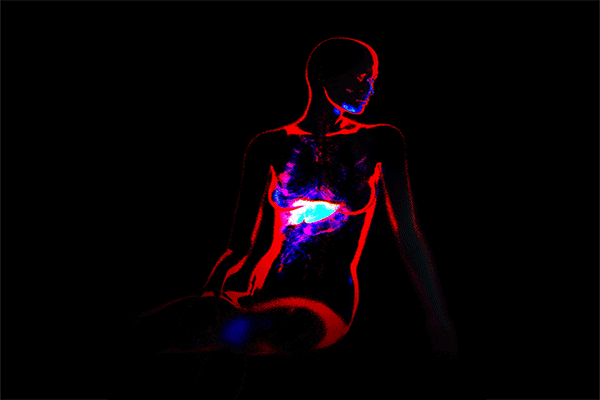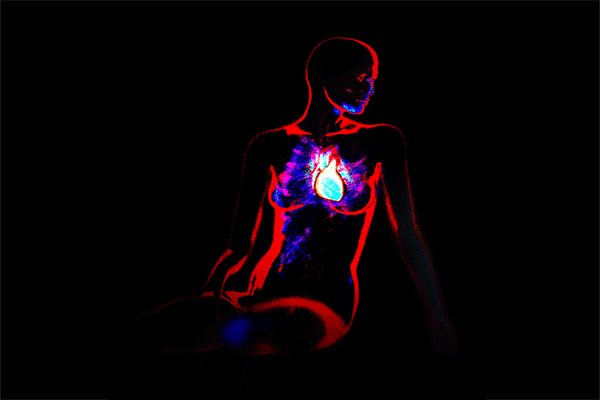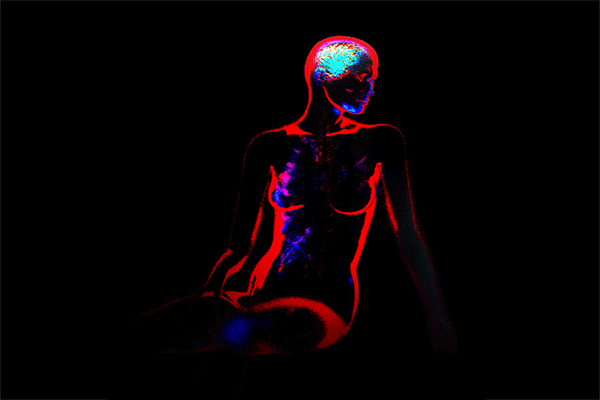
Straight to the Heart, Lungs, Kidney, or Brain

The broad category of long COVID could include people who have sustained damage from SARS-CoV-2 infection to specific organs in the body such as the heart, lungs, kidney, or brain.
Sometimes it is not crystal clear how someone’s symptoms are directly related to coronavirus infection.
Jennifer Fagan, a scientist and mother of two, caught what she now assumes was COVID-19 in March 2020, just as testing was becoming available at Emory.
She developed a mild fever, and that’s when she went in for a test, which was negative. But a few days later, her fever intensified, up to 104 degrees. Several years ago, while working in West Africa as a public health scientist, Fagan had malaria—but this was worse, she says.
Along with painful breathing and chest pain lasting a month, she had a skin rash, diarrhea, and eye irritation (conjunctivitis). However, Fagan did not come to the point of needing to go to the hospital. Her primary care physician, Matthew Marchal, diagnosed her with COVID-19 based on her symptoms and their severity.
“In April, we had many patients that were ‘test negative, presumed positive,’ ” Marchal says.
What happened to Fagan developed insidiously then manifested suddenly. In pre-COVID times, she practiced yoga and enjoyed running three times a week, at one point completing a half marathon. That fall, if she tried to go for a jog, she would feel dizzy and her heart would race, and yoga felt impossible. She assumed that part of what she experienced was deconditioning: being out of shape after so much time not exercising. But along with episodic dizziness and dramatic changes in her heart rate, she noticed other symptoms—persistent skin irritation and patches of hair loss (alopecia).
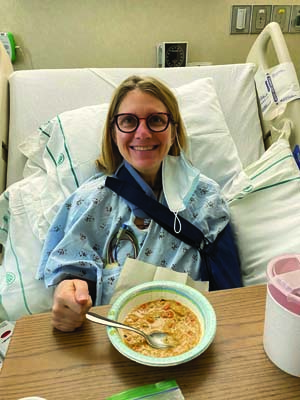
Jennifer Fagan, in the hospital.
She was slowly able to get back to jogging a few miles again. But in December, while sitting on a couch having breakfast, Fagan went into cardiac arrest.
She was in North Carolina with her family, and survived partly because her husband had taken a course in CPR. He started performing chest compressions, while her teenage daughter called an ambulance.
“My husband initially thought I was having a seizure,” she says. “He told me that my face turned yellow. After a while, my eyes popped open, and I started breathing again.”
In the emergency department of a North Carolina hospital, doctors could see that she had abnormal heart rhythms. They diagnosed her with myocarditis, inflammation of the heart muscle, and implanted a defibrillator.
Afterward, at home in Georgia, Fagan felt unsteady and unable to work.
On clear days this winter, she would occasionally go walking in her neighborhood, asking a friend to come with her just in case. “I’ve got my babysitter with me,” she would say ruefully.
Fagan consulted a cardiologist, who performed several diagnostic tests, looking for abnormalities.
The cardiologist examined her heart with ultrasound and she underwent a stress test, in which her heart rhythms were monitored.
She also visited a pulmonologist, who had her breathe into a tube to test her lung function.
Nothing stuck out.
Fagan was only diagnosed later with myocarditis based on cardiac MRI, which is more sensitive than other tests. Her experience may be an outlier.
Emory cardiologist Jonathan Kim, who advises Atlanta sports teams, has concluded that amateur athletes can generally return to exercise safely after having COVID-19—if they don’t experience cardiac-related symptoms— without undergoing a cardiac MRI. He adds, however, that “all athletes post-COVID-19 infection should have a slow and gradual return to training with close monitoring of persistent symptoms, regardless of the severity of infection.”
Still, a postinfection inflammation of the heart, possibly exacerbated by an autoimmune reaction, could explain a slowly developing problem like Fagan’s. “It’s a plausible mechanism,” says Emory cardiology researcher Ahsan Husain, who studies cardiomyopathy.
Meanwhile, Fagan is left with more questions than answers. “How do you know what’s relevant and how it fits together?” she asks.
Indeed, say Emory clinician researchers, much more about long COVID and its impact on the body remains to be discovered.
Email the Editor