Look Into My Eyes
The eye is a transparent sphere, a microcosm of the body whole. Its transparency offers doctors and researchers an unobstructed view of blood vessels, nerves, and connecting tissues. By analyzing subtle ocular changes, researchers can detect early signs of systemic diseases. This has created an emerging field of study—oculomics, a science so new it didn’t have a name until 2020.
LIUBOV MAHDA
Ophthalmologist Sruthi Arepalli and biomedical engineer Sudeshna Sil Kar embody the interdisciplinary nature of oculomics. They worked together to make pathbreaking discoveries about Alzheimer’s disease, an ailment completely outside their respective specialties.
“I’ve always been a little interested in Alzheimer’s,” says Arepalli, assistant professor of ophthalmology in Emory School of Medicine. “My mom works at the National Institutes of Health in the National Institute of Aging. I grew up hearing about these diseases.”
Sil Kar, assistant scientist in Emory School of Medicine, became invested when her father was diagnosed with Alzheimer’s. “When the disease was detected, it was too late because there is no treatment for this,” she says. “I wanted to know whether we could detect the disease earlier.”
Eyes provide clues to diseases

Sruthi Arepalli, assistant professor of ophthalmology at Emory School of Medicine.

Sudeshna Sil Kar, assistant scientist at Emory School of Medicine
“We see a lot of diseases in the retina: diabetes, hypertension, lymphoma, multiple sclerosis, lupus. All these things can impact the retina in predictable patterns. You’re sort of like Sherlock Holmes.”
“The retina is the window of the brain,” Sil Kar notes, “because it is connected to the brain through the optic nerve. We tried to find the association between retina structural changes, vascular changes, and Alzheimer’s disease.”
Finding innovations through AI
Arepalli and Sil Kar collaborated with Anant Madabhushi, director of the university-wide Emory Empathetic AI for Health Institute, to look for subtle changes in the shape of retinal blood vessels that could be correlated with other markers of Alzheimer’s.

Rohan Dhamdhere, doctoral student in Emory School of Medicine
In a new study, Arepalli, Sil Kar, and collaborators examined the network of vessels in the retinas of people with Alzheimer’s, along with retinas of healthy individuals.

Amrit Singh, graduate research assistant in Emory School of Medicine
With further validation, their discovery could lead to clinical tests predicting Alzheimer’s earlier and more accurately than current tests.
But the technique isn’t limited to Alzheimer’s.
Madabhushi believes many current medical diagnostic techniques will benefit from AI’s greater analytical powers.
“It’s almost like you’re using just 1 percent of the total amount of data available in the image and largely ignoring the rest of it,” he says. “AI is now scouring the remaining 99 percent of the data and trying to find and unearth more useful patterns.” “It’s ripe with potential,” Arepalli adds. “There are a lot of subtle changes within organ systems that we’re not able to appreciate in the same way that AI can.”
Recently, two other researchers working with Madabhushi found that changes in retinal blood vessels showed signs of being early predictors of heart disease.
Doctoral student Rohan Dhamdhere studied chronic kidney disease patients, who often have a higher risk of cardiovascular disease. “Early signs of issues with the heart can be seen in smaller vessels,” he says. “The circulatory system of the eye is the same as the heart. So retinal images are a very good way to look at vascular changes and effectively predict if there are going to be future changes or risk of cardiovascular disease.”
Graduate student Amrit Singh, who studied timing of major cardiovascular events in healthy patients, noted that heart diseases involve blockage of the arterial walls.
“Either a block deposition or hardening of the vessels,” he says. “So it was natural, given that we have retinal imaging, to see whether the changes would start in the smaller vessels.”
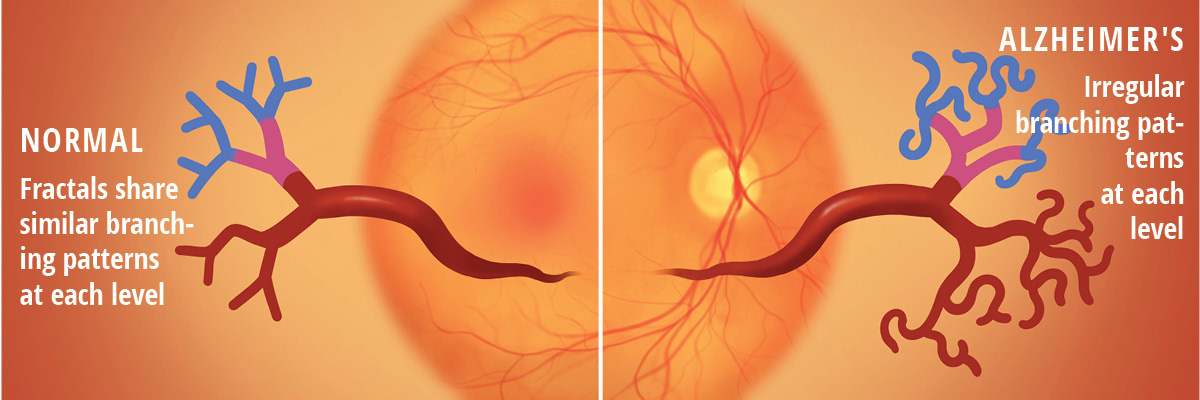
ILLUSTRATION: MICHAEL KONOMOS MEDICAL ILLUSTRATOR ESOM
In their separate studies, both researchers used AI models to analyze the micro vessels in their subjects’ retinas, looking for different patterns of twisting and curvature in their retinal arteries as well as different patterns of branching off from the main vessel.
Dhamdhere and his collaborators used a deep learning model to analyze the retinas of 811 patients with chronic kidney disease. Their eye-based model for coronary risk outperformed an existing, widely used risk model based on traditional measures such as cholesterol levels, blood pressure, and body mass.
Singh and colleagues, studying 2,120 patients with no prior history of cardiovascular disease, also found their model predicted risk better than another existing risk model that incorporated factors such as age, sex, race, cholesterol level, and smoking. With further validation, he thinks this procedure could become a routine screening tool used in medical exams.
Empowering physicians
“Everyone goes to a primary care physician,” he observes. “So empowering primary care physicians with this tool can help earlier screening of high-risk patients, which is currently not being done.”
Despite these innovations, Madabhushi thinks medical images are still being underused as a diagnostic tool, making them a continuing target for the insights AI models can provide.
His team is investigating whether retinal images also can produce insights into other diseases such as Parkinson’s and diabetes, working in close collaboration with medical specialists. “I know who I am and what I know,” he says. “I’m proud of the fact I’m a biomedical engineer, but I also appreciate that I don’t have a degree in medicine. But I am willing and eager to learn.”
When all the stakeholders go in with that attitude, Madabhushi says, amazing things can happen. “The greatest compliment I can get when I give a talk about my research and somebody asks, ’At which hospital do you practice pathology? Or radiology?’ Of course, I’m none of those things, but it clearly reflects that I’m willing to make the effort to truly understand other domains.”
This range of new possibilities convinces Emory’s researchers that the full power of retinal imagery is only beginning to be discovered. “There’s tremendous excitement about the ability of machine learning to recognize patterns that aren’t always apparent to the human eye or even to human judgment,” says Madabhushi. “It’s a great example of where the actual diagnostic information is highly underused.”
“We’re looking at how retinal imaging can be helpful for overall systemic health,” Amrit Singh agrees. “That includes not only cardiovascular diseases, but neurodegenerative diseases for, say, Parkinson’s and Alzheimer’s and maybe also the spectrum of cancer.”
Can Your Pupils Predict Alzheimer's?

LIUBOV MAHDA
The research suggests that pupil movements could help track activity in a key brain region called the locus coeruleus, one of the first areas affected by Alzheimer’s. “Findings point to the potential development of novel biomarkers for detecting early changes in Alzheimer’s,” says Deqiang Qiu, co-director of the Biomarker Core for Imaging at Goizueta Alzheimer’s Disease Research Center.
Researchers identified a network of brain regions connected to pupil activity, showing that this network weakens with age, which impacts memory, problem-solving skills, and spatial awareness. Higher levels of a protein called tau—an early sign of Alzheimer’s— were closely connected to these changes.
Email the Editor